New Discovery Challenges Established Laws of Thermodynamics

In a groundbreaking revelation that has captured the attention of the scientific community, a team led by graduate student Anthony Raykh at the University of Massachusetts Amherst has uncovered a phenomenon that defies the established Laws of Thermodynamics. This unexpected finding involves two immiscible liquids being influenced by magnetized particles, leading to results that challenge conventional understanding of liquid interactions.
Traditionally, the Laws of Thermodynamics detail how temperature, energy, and entropy interact within a system, providing a framework for explaining processes such as emulsification. Emulsification is the method by which two liquids that do not naturally mix, such as oil and water, can be combined into a stable mixture. A common example of this is seen in peanut butter, where the oil tends to separate, creating a top layer that must be mixed back in before consumption. To prevent this separation, many food companies add emulsifiers, which work to stabilize the mixture, all of which is governed by thermodynamic principles.
As Thomas Russell, a senior author on the research paper published in the journal Nature Physics, explains, âImagine your favorite Italian salad dressing. Itâs made up of oil, water, and spices, and before you pour it onto your salad, you shake it up so that all the ingredients mix.â This illustrates the process of emulsification that we often take for granted.
However, the dynamics took a bizarre turn when Raykh and his team mixed a combination of immiscible liquids along with magnetized nickel particles in a laboratory experiment. Instead of achieving the expected outcome of a homogeneous mixture, the result was a formation that resembled a Grecian urn shape. This unexpected outcome led Raykh to seek insights from his professors and collaborate with scientists from Syracuse University and Tufts University.
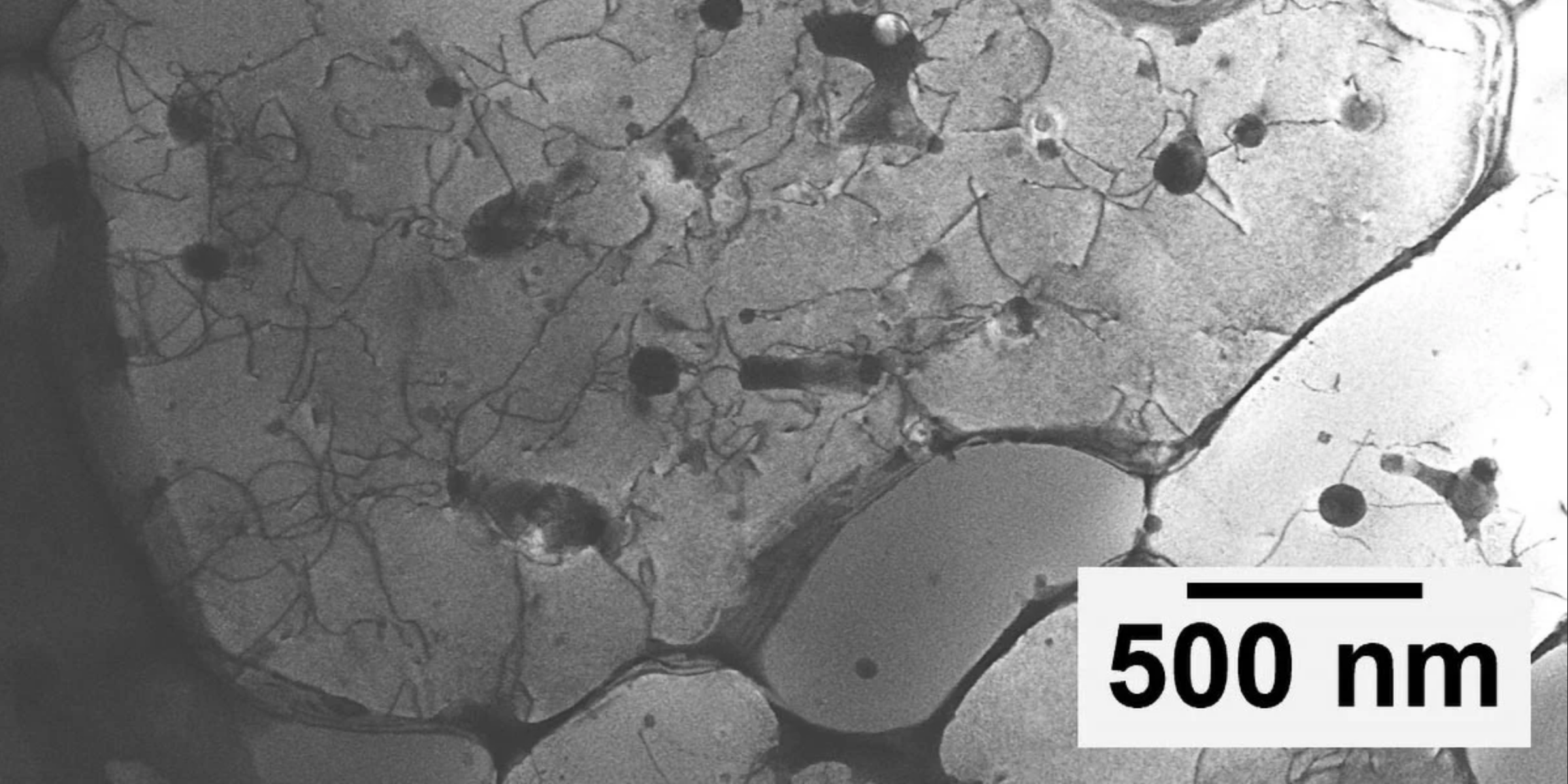
Through detailed simulations, it was discovered that when the magnetic influence on the liquids was sufficiently strong, it could curve the interface between the two liquids and disrupt the typical emulsification process dictated by thermodynamic laws. Even vigorous shaking could not alter the persistent Grecian urn formation, which raises intriguing questions about the behavior of liquids under unique conditions.
Hoagland, another researcher involved in the study, remarked on the intricate details observed at the boundary created by the magnetized nickel nanoparticles. âWhen you look very closely at the individual nanoparticles of magnetized nickel that form the boundary between the water and oil, you can get extremely detailed information on how different forms assemble. In this case, the particles are magnetized strongly enough that their assembly interferes with the process of emulsification, which the laws of thermodynamics describe,â he stated.
While Raykh acknowledges that this discovery does not currently lend itself to practical applications, it represents a novel state that has yet to be seen in the realm of soft-matter physics. The implications of this finding could potentially lead to a deeper understanding of liquid dynamics and inspire future research avenues.
As science continues to evolve, such discoveries remind us that there is still much to explore, even in well-established fields like thermodynamics.