Novel Pain Pathway Model Created in Laboratory to Enhance Analgesic Drug Development
In a groundbreaking advancement in neuroscience, researchers at Stanford University have successfully recreated a pain pathway in the brain by cultivating four essential clusters of human nerve cells in a laboratory setting. This innovative model, referred to as a brain assembloid, has been designed to not only elucidate certain pain syndromes but also to provide a new methodology for testing potential analgesic drugs, as reported in the prestigious journal Nature.
Dr. Stephen Waxman, a professor at Yale School of Medicine and not a participant in the study, expressed his enthusiasm about the research, stating, Its exciting. His comments highlight the significance of such advances, given that traditional methods for testing prospective pain medications often rely on animal models. These animal responses can differ significantly from human reactions, and individual nerve cells tested in isolation may not accurately represent the complex behaviors of entire brain networks.
With the advent of the brain assembloid, researchers now have access to a miniature nervous system that could serve as a versatile platform for deeper exploration of pain mechanisms. Dr. Sergiu Paca, a professor at Stanford and the lead investigator of this project, explains that their model aims to replicate the intricate signaling chain that occurs when one encounters painful stimuli. For instance, when a person accidentally touches a hot stove, specialized cells in the skin send a signal up to the spinal cord, which then relays the information to the thalamusdeep within the brainand ultimately reaches the outer layer of the brain known as the cortex.
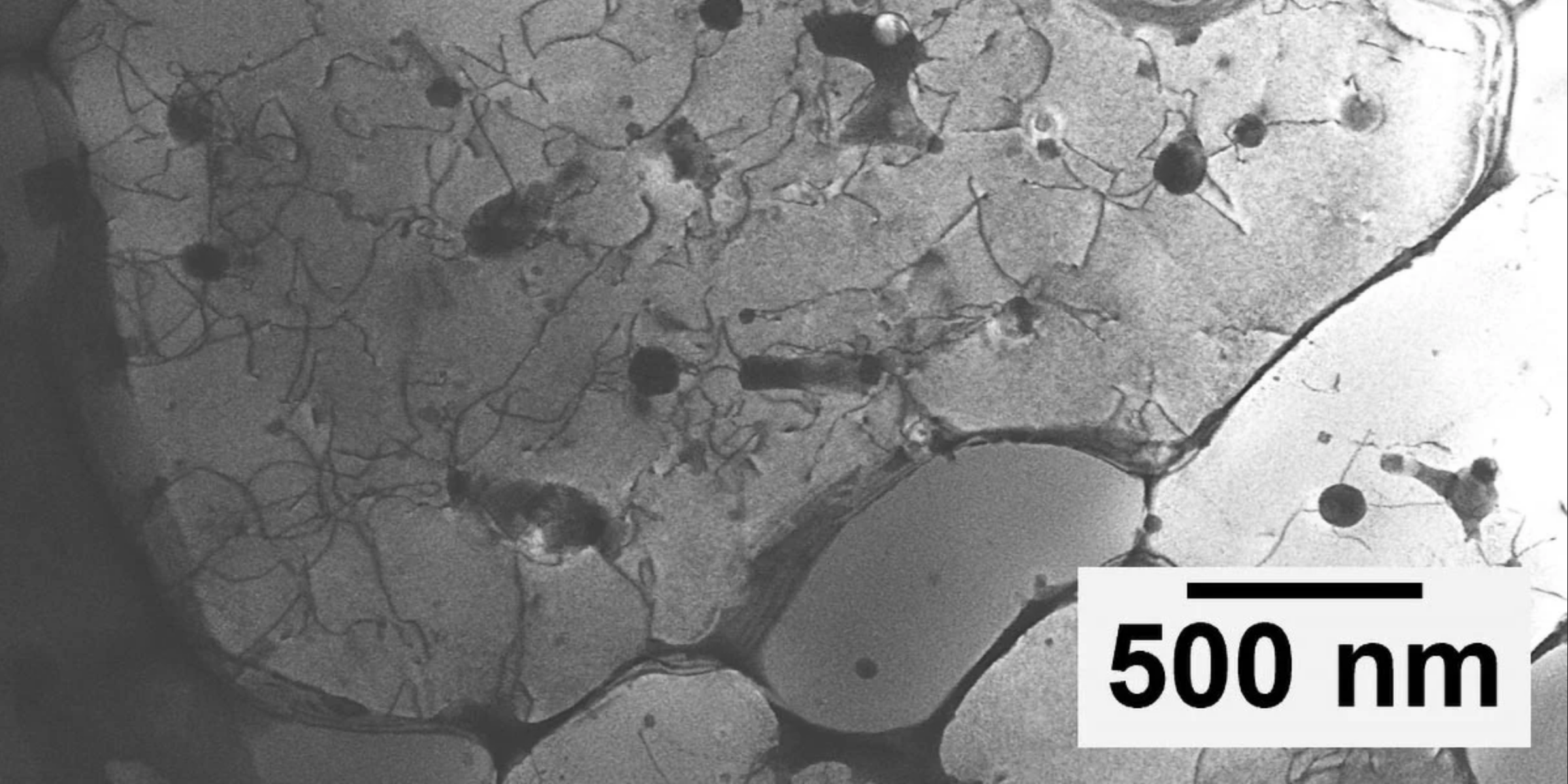
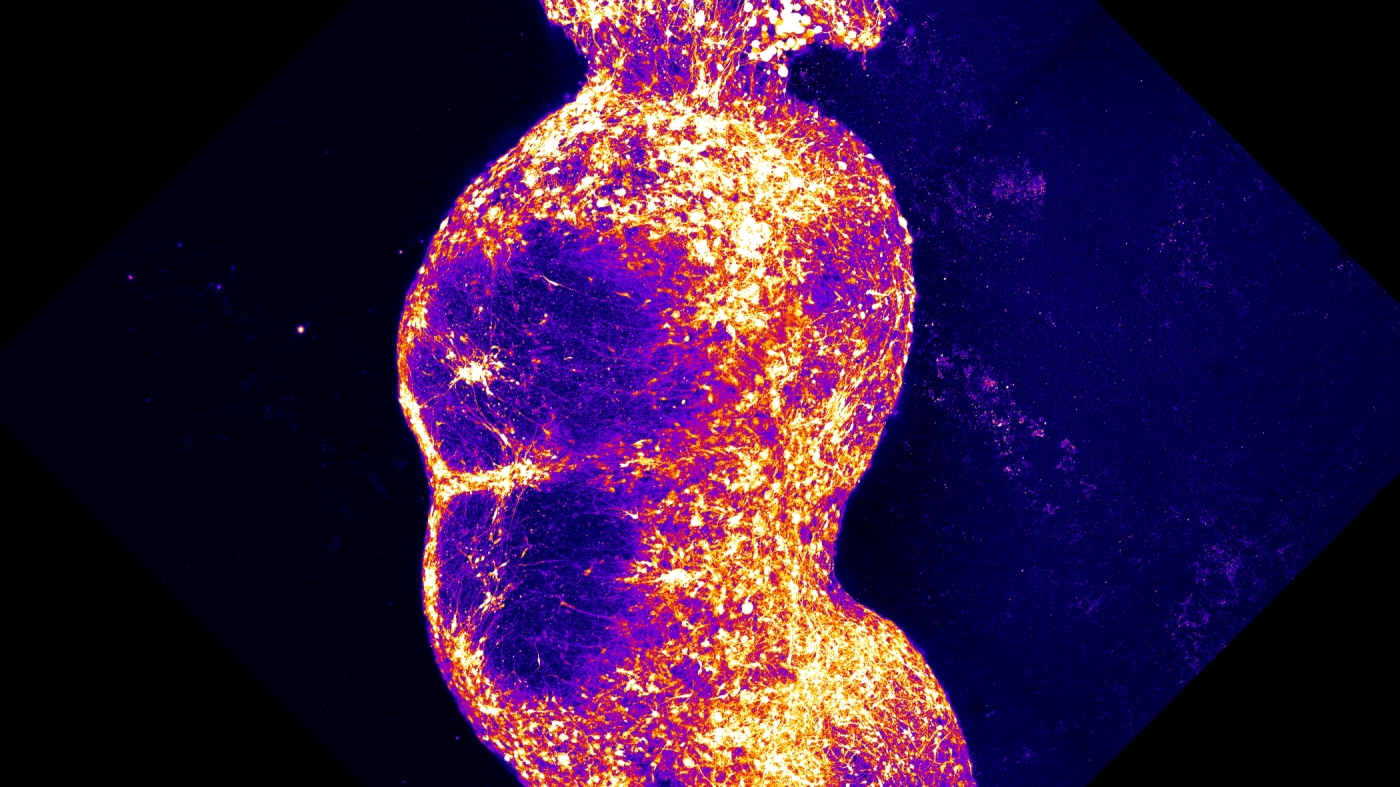
To effectively mimic this pathway within the laboratory, Pacas team developed four distinct brain organoidsspherical aggregates of human nerve cells that grow in culture. Each organoid was carefully manipulated to resemble a specific type of brain or spinal tissue associated with the pain pathway. We placed them in close proximity to one another and observed how they began to connect, Paca elaborated on their process.
After months of cultivation in the lab, the assembled organoids formed a functional pathway that effectively linked the four clusters. Remarkably, the nerve cells within the assembloid began to engage in coordinated activity across all four sections, demonstrating an interconnectedness that is critical for understanding pain transmission.
To test the efficacy of their model, the researchers introduced capsaicinthe active chemical in chili peppers known for its ability to induce a burning sensation. You can observe the transmission of information, said Paca. The neurons that detect these signals become activated, sending messages from one organoid to the next, culminating in a response that reaches the cortex.
Further experimentation involved creating assembloids using cells with genetic variants associated with atypical pain perception. One such variant is linked to a rare condition known as erythromelalgia, colloquially termed "man-on-fire syndrome." Individuals afflicted with this condition experience intense burning pain in response to mild warmth. According to Dr. Waxman, these patients endure excruciating sensations that are disproportionate to the stimuli they encounter.
The study revealed that assembloids carrying the gene variant exhibited significantly more spontaneous communication between the organoids, suggesting an increased sensitivity to pain. Such findings indicate that these organoids can serve as a valuable tool for examining nervous system disorders and the corresponding pathways they impact, as noted by Dr. Guo-li Ming, a professor at the University of Pennsylvanias Perelman School of Medicine, who was not involved in the current research.
While the pain pathway model offers profound insights, it is important to acknowledge its limitations. Dr. Ming points out that despite its complexity, this laboratory representation is a simplified version of the actual human pain pathway. Humans possess two major pathways for conveying pain signals to the brain, whereas the assembloid encompasses only one. Consequently, while the model can detect painful stimuli, it does not elicit an emotional response akin to actual pain experiences. We do not believe that this pathway weve constructed experiences pain in any way, Paca confirmed.
As research progresses, these clusters of human cells are expected to become increasingly instrumental as scientists endeavor to replicate more extensive and intricate sections of the nervous system. For instance, Dr. Mings laboratory has developed a model of a human neural tube, the embryonic structure that ultimately evolves into a babys brain and spinal cord. Her research aims to shed light on the developmental origins of neurological disorders.