Understanding Galvanic Corrosion in Water Cooling Loops: A Deep Dive

Water is widely recognized as an exceptional coolant, but it possesses another characteristic that can prove detrimental: it is also a highly effective solvent. This dual nature of water is the fundamental reason why any water cooling loop is susceptible to the phenomenon known as galvanic corrosion, a process that can lead to significant damage to components almost immediately. In a recent informative video produced by the renowned tech influencer [der8auer], this critical issue is vividly illustrated using a GPU cold plate, a component crafted from nickel-plated copper and designed with numerous small channels to maximize the surface area in contact with the coolant.
In theory, using distilled water in a cooling loop that contains only one type of metalsuch as coppershould not pose any major problems. However, as [der8auer] emphasizes, the reality is far more complex. In practical applications, components like fittings, radiators, and cooling blocks are typically constructed from a variety of metals and alloys, including brass. This mixed-metal setup creates an ideal environment for galvanic corrosion to occur. In this scenario, one metal will act as the anode while another will function as the cathode. While this behavior is advantageous in battery technology, it becomes problematic in cooling loops, where water aggressively strips metal ions off the anode and deposits them onto the cathode, leading to potential failures.
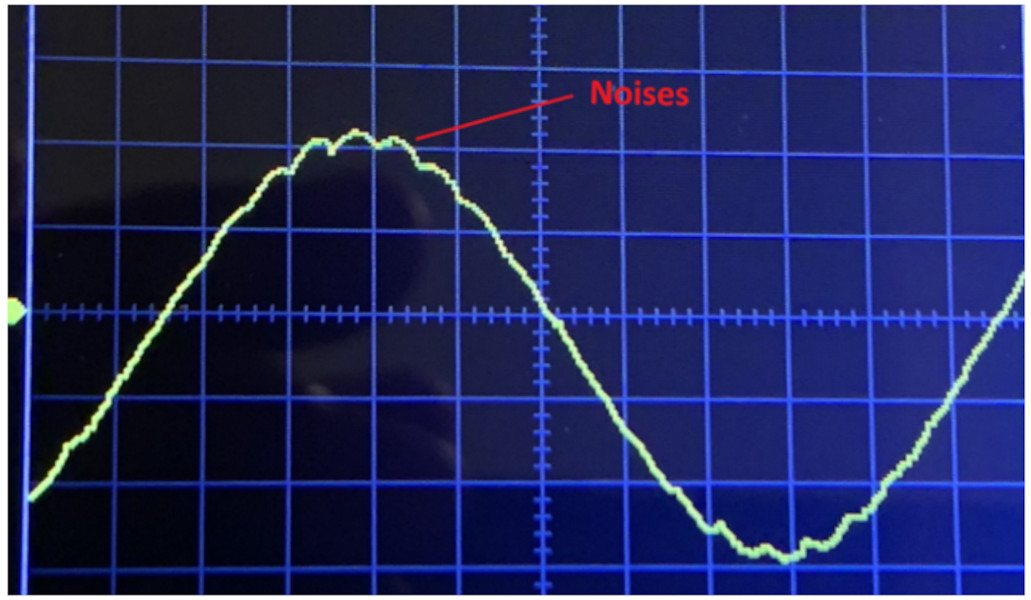
In an ideal world, a nickel-plated cold plate should resist galvanic corrosion if the nickel plating is flawless. Yet, as demonstrated in the video, even a brief contact with distilled water at a temperature of 60C resulted in pronounced galvanic corrosion. Advanced analysis utilizing Scanning Electron Microscopy (SEM) revealed that imperfections in the nickel plating allowed copper ions to dissolve into the water, which were then deposited on the nickel surface acting as the cathode.
Interestingly, the video also compared this scenario with another sample that utilized a coolant containing a corrosion inhibitor known as DP Ultra. In stark contrast to the first experiment, no signs of corrosion were detected in the second sample, even after extended exposure to similar conditions. The DP Ultra coolant is primarily composed of distilled water but incorporates glycol as an additive. Glycol serves to enhance the pH levels and coat the surfaces within the cooling loop to mitigate the risk of galvanic corrosion. Moreover, this coolant contains benzotriazole, a compound that further enhances protection against corrosion.
It is essential to note that each corrosion inhibitor is specifically designed to target particular environments, and challenges such as the formation of organic films may necessitate the addition of biocides to the system. As always, the intricacies of water cooling systems present more complexity than one might initially assume, and understanding these subtleties is crucial for anyone looking to maintain their systems effectively.















![Introducing Version 2 of the 3D Printed Perpetual Calendar Clock by [shiura]](https://hackaday.com/wp-content/uploads/2025/04/FN9CHN9M9E88YJ9.jpg)