This Single-Celled Microbe Can Transform Into a Multicellular Creature
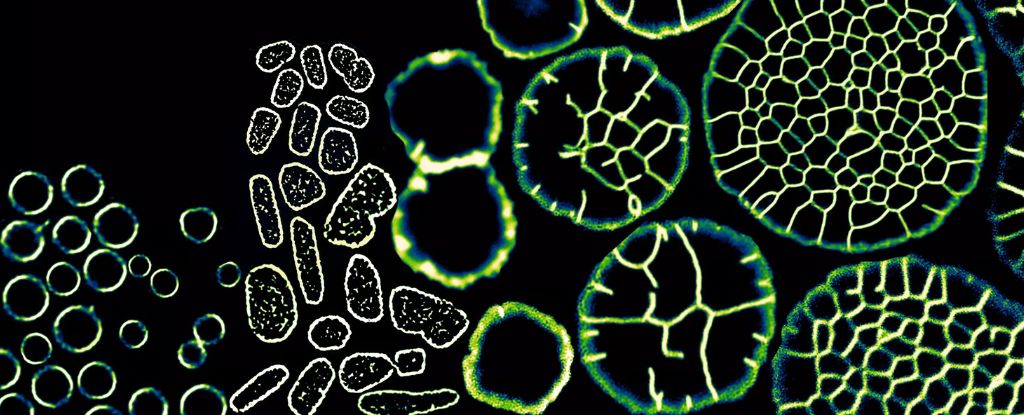
A single-celled microbe that revels in Earth's most hostile salt lakes has the remarkable ability to transform its mote of a body into multicellular tissue when the pressure's on. "The advent of clonal multicellularity is a critical evolutionary milestone," the international team who made this discovery, led by Brandeis University pathobiologist Theopi Rados, write in their new paper. Haloferax volcanii is a member of the often-overlooked archaea domain, which looks quite similar to bacteria and yet have more in common with our own domain, eukaryota. Multicellularity is common in eukaryotes and rare among bacteria, and as far as we know, H. volcanii is only the second archaeon found to take this multicellular leap. We know H. volcanii has some impressive shape-shifting techniques up its tiny sleeves to help it thrive in such extreme environments as the Dead Sea and the Great Salt Lake. When H. volcanii's outer layer is pulled taut by physical forces, Rados and her team found, the microbe takes on a form even more reminiscent of complex organisms: it goes multicellular. Rados first stumbled across this strange new strategy by tucking a single H. volcanii cell under a pad of jelly, which applied just 10 kPa of pressure – roughly what you experience at one meter below water. This weighted jelly blanket flattened the malleable cell in around two and a half hours, before the microbe even had a chance to clone itself. To see what would happen under forces more similar to the microbe's natural habitat, the researchers next placed H. volcanii under pressure of more than 100 kPa, which is equivalent to conditions around 10 meters underwater. Not only did the organism flatten like a pancake, but over 12 hours its cells, each containing multiple sets of genetic information, grew larger and organized into a fused cluster resembling the tissue of multicellular organisms. The microbes' flexible proteinaceous surface layer, more similar to animal cell membranes than the rigid cell walls of plants and fungi, seems key to its metamorphic ways. frameborder="0″ allow="accelerometer; autoplay; clipboard-write; encrypted-media; gyroscope; picture-in-picture; web-share" referrerpolicy="strict-origin-when-cross-origin" allowfullscreen> "The absence of a covalent-bound cell wall suggests a more dynamic, but less rigid structure, leading to the hypothesis that archaea might be 'squishy' and sensitive to mechanical stimuli," says Brandeis University archaea biologist, Alex Bisson. "It's as if the cells were squished down and then encouraged to grow wider and taller, more like a rising sourdough loaf than traditional cell division." The resulting tissues have physical properties distinct from the microbe's single-celled form, with elasticity between cells comparable to that of animal cells. That tension creates two different cell types in a layout reminiscent of a turtle's shell: wedge-like peripheral cells, named as such because they form at the edge of the tissue, are flatter and wider, while tightly-packed geometric scutoid cells are taller. The scutoid cells are most reminiscent of eukaryote bodies, where cells of this shape abound in the curves of epithelial tissue (like the surfaces of our gut and our skin) to evenly distribute membrane tension. Finding these shapes in an organism whose body plan predates eukaryotes suggests that scutoid cells might be older – and more fundamental to multicellularity – than we realized. "The fact that archaea can orchestrate complex tissue-like structures suggests that nature can emerge complex traits from seemingly unsophisticated raw materials," says Bisson. This research was published in Cell Biology, alongside a related perspective.



















